介紹
水簇是兩個或多個水分子通過氫鍵組裝形成的具有特定構型和拓撲模式的聚集體。水在許多生命和化學過程中起著十分重要的作用。水的化學組成十分簡單:一個氧原子和兩個氫原子所組成的分子型化合物。然而它卻體現出很多不同於其他化合物的物理化學性質。例如,根據熱力學計算,以單分子存在的水其熔點和沸點應是-110 C和-85 C,遠小於實際的0 C和100 C,由於水異常的熔沸點等特殊性質,人們推測在自然狀態下水並非以單分子存在,而應以分子簇的形式存在。但直到1977年,Deky等利用紅外光譜測試儀才首次證實了二元水簇的存在。
水是大量水分子的氫鍵聚集體。每一個水分子有兩個氫原子和兩對孤對電子,可以與其相鄰的水分子形成二個、三個或四個氫鍵。這些氫鍵作用的詳細結構信息是水的研究的關鍵之一。然而,由於水的微觀複雜性,難以量化其結構信息,這給水的定量研究帶來很大困難。水簇作為大量液態水的簡化,可以為液體水性質的研究提供有效的途徑。水簇的研究不僅可以了解水的一些不同尋常的行為,還為了探索水分子在生物體內的存在形式與所起到的作用以及設計新穎的功能材料。同時,水簇的研究也有助於揭開諸如酸雨的形成、雲層的吸收和雨滴的成核等眾多自然現象的謎團。
人們通過理論模擬和光譜實驗方法對水簇進行了研究,可以揭示液態水分子簇存在的結構、穩定能量、力學機制等特點。近年來,隨著X-射線衍射技術的進步,人們可以通過單晶衍射儀精確地測定晶格中水分子的位置,從而精確的描述水分子之間的氫鍵作用的各項參數,能夠更清楚的了解水的各種物理化學行為。一般來說,水分子簇不可能孤立的存在,而是作為客體分子存在於某一種主體結構中。
不同結構
截至目前為止,已有很多例不同結構的水簇被表征,按照構成水簇的水分子個數,水簇可以分為小分子水簇、一維水簇、二維水簇和三維水簇。
小分子水簇
小分子水簇也稱低聚水簇,指的是由個數有限的水分子通過氫鍵形成孤立存在的簇狀聚集體,簇與簇之間並沒有通過氫鍵相連。
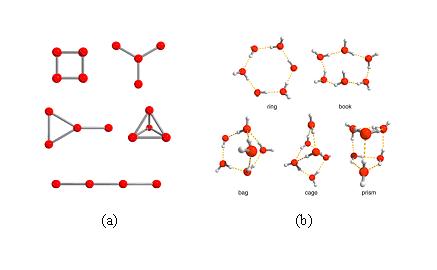
在目前所報導的小分子水簇中,被關注的較多是四元水簇與六元水簇。四元水簇是液態水和固態凍的二維模型中最簡單的特例。如圖1(a)所示,由於氫鍵的模式不同,四個水分子具有五種不同的連線模式,包括環狀、心形、手鼓形、四面體狀和線形。其中四元水環的能量更低,也更容易形成,因此所報導的四元水簇中,四元環狀模式是最常見的。而與四元水簇不同,六元水簇代表了水簇從二維跨入三維。理論計算表明六元水分子簇可以有環狀、書狀、袋裝、籠狀和稜柱狀等幾種構型,其能量差異在0.7kcal/mol以內,如圖1(b)所示。籠狀是能量最低最穩定的一種構型,隨後是書狀和稜柱狀,它們比籠狀分別高0.1和0.2 kcal/mol;能量較高的是環狀與袋裝,它們比籠狀分別高0.5 和0.7 kcal/mol。在所報導的水簇合物中,前兩種居多,特別是以環狀六元水簇最為常見。
圖1 (a) 四元水簇的五種不同的連線模式(氫原子被忽略);
(b) 六元水簇能量較低的五種構型
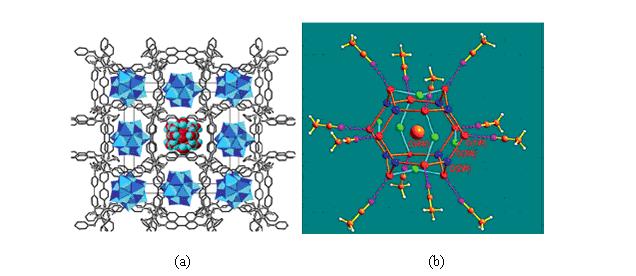
除了(H2O)4和(H2O)6以外,已有報導的小分子水簇還有(H2O)2 、(H2O)3 、(H2O)5 、(H2O)7 、(H2O)8 、(H2O)9 、(H2O)10 、(H2O)11 、(H2O)12 、(H2O)14 、(H2O)16 、(H2O)17 、(H2O)18 、(H2O)26 、(H2O)27等等。目前報導的最大的小分子水簇(H2O)27是在配合物[Co4(dpdo)12][H(H2O)27(CH3CN)12][PW12O40]3中發現的,它以質子化的形式H(H2O)27存在。如圖2所示,[Co4(dpdo)12]和[PW12O40]離子形成一具有空洞的三維骨架,每一個空洞中,共存有12個(CH3CN)12分子和27個結晶水分子。其中26個水分子通過氫鍵相連形成(H2O)26籠狀水簇。而剩下的一個水分子(O4W)進入(H2O)26水籠中並質子化,形成H(H2O)27離子。
圖2. (a) H(H2O)27通過氫鍵主體與連線; (b) H(H2O)27內部連線模式
一維水簇
一維水簇介於二維水簇和小分子水簇之間,可以認為是二維水簇的最簡模型,同時也比小分子水簇更接近於固態冰或液態水的結構。它在某些生命和化學過程扮演重要角色。一維水簇可以分為水鏈、水帶和水柱。一般水鏈指水分子首尾直接相連而形成的一維水簇,而水帶則由一種或幾種環狀小分子水簇通過共用水分子相連而成,除一維無限外,另一維具有一定的寬度;如果水分子排列時,一維無限同時另兩維均具有一定寬度,則稱之為水柱。不過,由於近年所報導的一維水簇中存在不少結構比較複雜難以歸屬的模式,所以這樣的分類其實是比較模糊的。
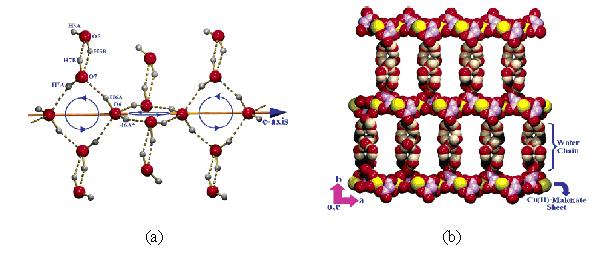
圖3. (a) 一維帶冠四聚體螺旋水帶的內部連線模式;(b) 一維帶冠四聚體螺旋水帶連線主體的二維配位聚合層形成磚牆式超分子結構。
許多一維水簇顯示了精美的結構。例如,A. D. Jana等人製備了配聚物{[Cu(mal)2](picH)2·5H2O}n(mal為丙二酸,pic為2 -氨基- 4 -甲基吡啶),其中含有一個帶冠的四聚體螺旋水帶。如圖3所示,在該一維水簇中,相鄰的四元水環通過共用一個水分子(O6)並扭轉成86.44°的夾角相連,沿著c軸形成一維帶狀結構,而每個四元水環上下各接一個水分子(O5)形成冠狀結構。而銅離子和丙二酸根通過配位間形成二維層狀,該一維水簇通過氫鍵連線相鄰的二維層形成三維的磚牆式超分子結構。
二維水簇
從結構上看,二維水簇比一維水簇更接近於大量液態水或固態冰。例如在配合物Cd(H2O)2Ni(CN)4·4H2O存在二維水層,水分子中O···O的距離為2.821(9)和2.86(2) Å,與液態水的相應距離相當(2.85Å)。而在有機水合物bpedo·5H2O(bpdeo為反式-1,2-雙(4-氧化吡啶基)-乙烯)存波浪形的在二維水層,其中O···O的平均距離為2.748 Å (90K)和2.776 Å (295K),非常接近於固體冰Ih中的相應距離(2.759 Å)。
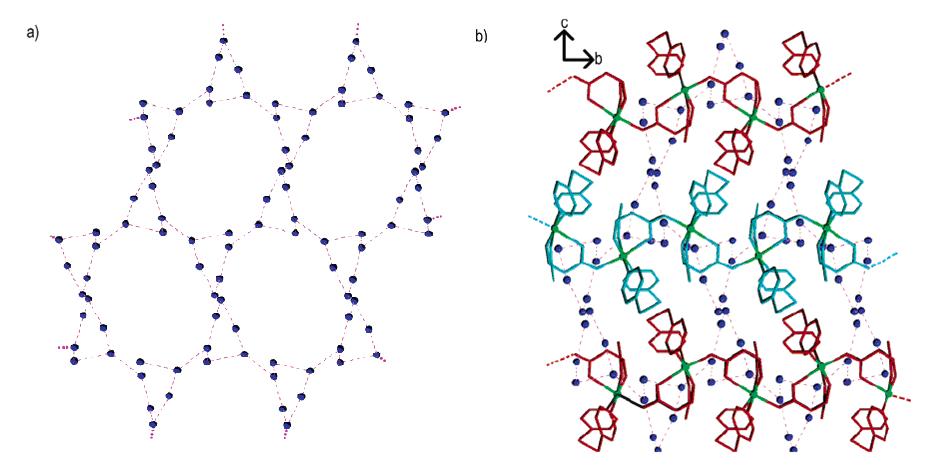
某些二維水簇中可能形成很大的孔穴,如R. Carballo等報導了含有(H2O)18水環的大孔水層結構。如圖4所示,該水層存在於配位聚合物[Cu(Hmal)(4pds)] ·6H2O中(4pds為4,4’-二硫代聯吡啶,mal為蘋果酸),水層中含有有18個水分子形成的巨大水環,其平均孔徑達11.76 Å。該大孔水環和[Cu(Hmal)(4pds)]的二維配聚物主體形成了相互穿插的超分子結構。
圖4. (a) 含有(H2O)18水環的二維水簇; (b) 二維水簇和主體相互穿插。
三維水簇
三維水簇十分罕見,圖5為僅有報導的三維水簇示意圖。此三維水簇存在於下面的離子配合物中:[Zn(HL)(phen)2][Zn(L)(HL)(phen)]·13H2O [H2L = 甲基乳酸(methyllactic acid);phen = 1,10-鄰菲囉啉]。在此配合物中包含[Zn(L)(HL)(phen)]負離子, [Zn(HL)(phen)2]正離子和13個結晶水分子,兩種不同的陰陽離子通過H鍵和π-π相互作用形成一維鏈,誘導水分子分布於其周圍形成3D水簇結構。
圖5. 在[Zn(HL)(phen)2][Zn(L)(HL)(phen)]·13H2O中三維水簇
水簇拓撲模式
隨著越來越多的水簇被測定與表征,人們開始考慮如何來界定和描述水簇內部水分子的連線方式。L. Infantes等人重新檢視劍橋晶體學資料庫(CCDC)中的結構,發現在先前所合成的許多水合物中同樣包含有各式各樣的水簇,但這些化合物在最初報導時並沒有提及水簇的存在。基於對CCDC資料庫中水簇模式的分析,他們提出採用拓撲模型來描述水簇,這一方法主要將水簇分為五種不同的模式:(a)離散鏈狀,代號為Dn,其中D為Discrete Chains,n為水簇內所形成的鏈具有的最大水分子個數。在Dn中,水分子呈線性連線模式,不包含環,同時也不是無限連線的;如果水分子存在分叉,則以所形成的最長鏈的水分子個數計為n。(b)離散環狀,代號為Rn,其中R為Discrete Rings,n為水簇內所形成的環具有的最大水分子個數。在Rn中,水分子也是有限連線的,形成單個的環狀形式。(c)一維無限鏈狀,代號為Cn,其中C為Infinite Chains,n為所形成的一維無限鏈最小重複單元的水分子個數。(d)一維無限帶狀,代號為Tn(m)Ak,其中T為Infinite Tapes,n為形成的環所包含的水分子個數,m為環與環之間共用的水分子個數,Ak指無交集環之間所間隔的水分子個數。(e)二維無限層狀,代號為Lm(r)n(s)···,其中L為Infinite Layers,m,n為平面內形成的環包含的水分子個數,r,s為環外與之有共用水分子的環的個數。
上述五種模式中,a、b為小分子水簇,c、d為一維水簇,e為二維水簇。雖然它們是基於有機宿主中水簇分析後得到的,但應可以被套用到不同主體中水簇的分析中。在前兩種模式中,即線形和環狀模式中,由於在定義水簇模式時,只考慮成鏈或成環所具有的最大水分子個數,因而會出現某些三元、四元和五元水簇均為D3模式,無法體現它們的差異。因此,袁良傑課題組提出了一種改進的命名規則:借鑑有機化合物中烷烴和環烷烴的命名規則,以最長的鏈或最大的環為水簇的主鏈(環),而其它的水分子則看成是取代基,引入m(r)n(s)···Dn和m(r)n(s)···Rn來修訂水簇的模式,這裡m,n為取代水分子在水簇的主鏈(環)的取代位置,r,s為取代水分子的個數。例如,上述三種D3模式的水簇依次可以分別定義為:D3、2(1)D3和2(1)2(1)D3。另外,這種修訂可以被擴展到一維鏈狀模式的水簇中。如一維C3水鏈中最小重複單元為三個水分子,如果在第一個水分子上連線一個水分子,則可以定義為1(1)C3。類似的,如果一個水分子連線與第二個水分子,則可以定義為2(1)C3;如果第一個和第三個水分子分別連線一個水分子,則可以定義為1(1)3(1)C3。儘管這種改進的命名規則能夠描述更多水簇的構成形式,但由於實際水分子連線模式的複雜性,隨著更多複雜的水簇被合成和表征,很多水簇無法用上述簡單的符號來定義。
主體設計
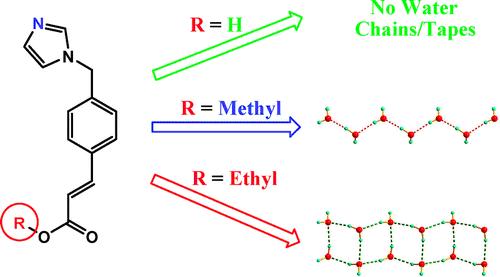
目前所報導的水簇大多存在於一些特殊的主體結構中,即水簇是作為主體結構的副產物被表征的,而關於水簇的結構與主體結構相關性的報導則較少涉及。例如圖6所示,在有機化合物3-(4 -(1 H -咪唑-1-基)甲基苯基)丙烯酸及其衍生酯類為主體的水合物中,若主體為羧酸時,不能形成水簇結構。而若羧基上的氫原子被甲基所取代時,形成一維C2水鏈結構。當羧基上的氫原子被乙基所取代時,形成一維T4(2)水帶結構。在這些水合物中,儘管主體的堆積模式相似,但由於取代基的不同,使得在主體堆積時所形成的一維納米孔道內部微環境不同,進而導致形成不同結構的一維水簇。這樣,通過調控主體有機分子上的取代基,就可以設計合成不同形貌的水簇結構。
圖6. 通過調控有機主體上的取代基獲得的不同一維水簇
中山大學的陳小明等通過通過選擇相似的次級結構單元即二聚金屬離子[Cd2(bpa)2]作為節點(bpa 為N,N’ -二(吡啶醯胺)吖嗪),以不同的羧酸配體作為連線,構築出4個具有不同拓撲結構的配位聚合物。在這些配位聚合物主體中,存在著結構各異的一維水簇。而這些不同結構水簇的形成依賴於合適的次級結構單元和不同有機羧酸配體連線所成的配位聚合物主體。
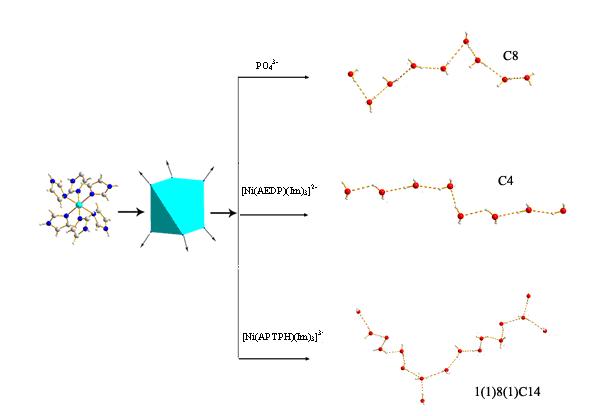
圖7 通過調節膦酸抗衡離子構造不同拓撲模式的一維水鏈
袁良傑課題組對以有機膦酸為主體構築的水簇結構進行了詳細研究,分別以磷酸、1-氨基-1, 1-乙叉雙膦酸(AEDPH4)和1-氨基-1, 1, 3-丙叉三膦酸(APTPH6)為前驅物,通過[Ni(Im)6]陽離子和不同的膦酸陰離子進行氫鍵自組裝,合成和表征了三種新的有機磷酸金屬配合物。從這三個配合物中分別得到不同的一維水鏈結構:C8螺旋狀水鏈,C4鋸齒狀水鏈,以及1(1)8(1)C14波狀水鏈。這樣,通過調節膦酸抗衡離子的結構,能獲得不同結構的主體,從而可以構造出具有不同拓撲模式的水簇(見圖7)。此外,陳碩平等首次發現了氨基雙膦酸在水熱條件和金屬離子的催化下發生原位縮合反應,獲得一類結構新穎的的雙輪膦酸配體,並以這類雙輪膦酸為前驅物,合成和表征了4個分別包含有複雜的一維T4(3)5(0)A0水柱、一維(H2O)24水柱、二維L5(4)5(5)15(14)水網和一維(H2O)36水柱的配合物。在配合物[Zn3(L1H)2(2,2'-bipy)3]·18H2O中,罕見的一維(H2O)36水柱和以雙輪膦酸為主體構築的一維配聚物鏈形成垂直穿插。由於雙輪膦酸較氨基雙膦酸有更多親水的膦氧基團,因此形成的水簇的拓撲模式更為複雜,另外通過局部調節配合物中的某一個離子或配體結構,也能夠改變水簇的拓撲模式。
參考文獻
[1] F. H. Stillinger. Water Revisited. Science 1980, 209, 451-457.
[2] T. R. Dyke. Group theoretical classification of the tunneling–rotational energy levels of water dimmer. J. Chem. Phys. 1977, 66, 492-497.
[3] J. M. Ugalde, I.,Alkorta, J. Elguero. Water Clusters: Towards an Understanding Based on First Principles of Their Static and Dynamic Properties. Angew. Chem. Int. Ed.2000, 39, 717-721.
[4] R. Ludwig. Water: From Clusters to the Bulk. Angew. Chem. Int. Ed.2001, 40, 1808-1827.
[5] R. M. Pitti, S. A. Marsters, D. A. Lawrence, M. Roy, F. C. Kischkel, P. Dowd, A. Huang, C. J. Donahue, S. W. Sherwood, D. T. Baldwin, P. J. Godowski, W. I. Wood, A. L. Gurney, K. J. Hillan, R. L. Cohen, A. D. Goddard, D. Botstein, A. Ashkenazi. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature 1998, 396, 699-703.
[6] SY. Lo, WC. Li, SH. Huang. Water clusters in life.Med. Hypotheses.2000, 54, 948-953.
[7]F. N. Keutsch, R. J. Saykally. Water clusters: Untangling the mysteries of the liquid, one molecule at a time. P. Natl. Acad. Sci. USA.2001, 98, 10533-10540.
[8] S. Vaitheeswaran, H. Yin, J. C. Rasaiah, G. Hummer. Water clusters in nonpolar cavities.P. Natl. Acad. Sci. USA. 2004, 101, 17002-17005.
[9] T. Ohba, H. Kanoh, K. Kaneko. Water Cluster Growth in Hydrophobic Solid Nanospaces. Chem. Eur. J. 2005, 11, 4890-4894.
[10]K. M. Jude, S. K. Wright, C. Tu, D. N. Silverman, R. E. Viola, D. W. Christianson. Crystal Structure of F65A/Y131C-Methylimidazole Carbonic Anhydrase V Reveals Architectural Features of an Engineered Proton Shuttle. Biochemistry 2002, 41, 2485-2491.
[11]H. R .Carton. Do clusters contribute to the Infrared absorption of water vapor? Infrared Phys. 1979, 19, 549-557.
[12]K. Nauta and R. E. Miller. Format ion of Cyclic Water Hexamer in Liquid Helium: The Smallest Piece of Ice. Science 2000, 287, 293-295.
[13] C. J. Gruenloh, J. R. Carney, C. A. Arrington, T. S. Zwier, S. Y. Fredericks, K. D. Jordan. Infrared Spectrum of a Molecular Ice Cube: The S4 and D2d Water Octamers in Benzene-(Water)8. Science 1997, 276, 1678-1681.
[14] K. Liu, J. D. Cruzan, R. J. Saykally. Water Clusters. Science 1996, 271, 929-933.
[15] J. Barciszewski, J. Jurczak, S. Porowski, T. Specht, V. A. Erdmann. The role of water structure in conformational changes of nucleic acids in ambient and high-pressure conditions. FEBS J. 1999 260, 293-307
[16] F. N. Keutsch, R. J. Saykally. Water clusters: Untangling the mysteries of the liquid, one molecule at a time. PNAS 2001, 98, 10533-10540
[17] G. Q. Jiang, J. F. Bai, H. Xing, Y. Z. Li, X. Z. You. A Tetrahedral Water Tetramer in a Zeolite-like Metal-Organic Framework Constructed from {(H3O)2[Fe6O{(OCH2)3CCH3}4Cl6]·4H2O}. Cryst. Growth Des. 2006, 6, 1264-1266.
[18] M. Zuhayra, W. U. Kampen, E. Henze, Z. Soti, L. Zsolnai, G. Huttner, F. Oberdorfer. A Planar Water Tetramer with Tetrahedrally Coordinated Water Embedded in a Hydrogen Bonding Network of [Tc4(CO)12-(μ3-OH)4·4H2O]. J. Am. Chem. Soc. 2006, 128, 424-425.
[19] L. S. Long, Y. R. Wu, R. B. Huang, L. S. Zheng. A well-resolved uudd cyclic water tetramer in the crystal host of [Cu(adipate)(4,4'-bipyridine)]·(H2O)2. Inorg. Chem. 2004, 43, 3798-3800.
[20] D. R. Turner, M. Henry, C. Wilkinson, G. J. Mclntyre, S. A. Mason, A. E. Goeta, J. W. Steed. Cooperative hydrogen-bonding effects in a water square: a single-crystal neutron and partial atomic charges and hardness analysis study. J. Am. Chem. Soc. 2005, 127, 11063-11074.
[21] J. Tao, Z. J. Ma, R. B. Huang, L. S. Zheng. Synthesis and characterization of a tetrazolate-bridged coordination framework encapsulating D2h-symmetric cyclic (H2O)4 cluster arrays. Inorg. Chem.2004, 43, 6133-6135.
[22] P. S. Lakshminarayanan, D. K. Kumar, P. Ghosh. Counteranion-controlled water cluster recognition in a protonated octaamino cryptand. Inorg. Chem.2004, 44, 7540-7546.
[23] M. T. Ng, T. C. Deivaraj, W. T. Klooster, G. J. McIntyre, J. J. Vittal. Hydrogen-bonded polyrotaxane-like structure containing cyclic (H2O)4 in [Zn(OAc)2(μ-bpe)]·2H2O: X-ray and neutron diffraction studies.Chem. Eur. J., 2004, 10, 5853-5859.
[24] S. Supriya, S. K. Das. A cyclic (H2O)4 cluster characterized in the solid state disappears on heating and regenerates from water vapor: a supramolecular reversible gas-solid reaction. New. J. Chem. 2003, 27, 1568-1574.
[25] S. Supriya, S. Manikumari, P. Raghavaiah, S. K. Das. A cyclic supramolecular (H2O)4 cluster in an unusual Fe3 complex that aggregates to{Fe3}n with a zig-zag chainlike structure. New. J. Chem. 2003, 27, 218-220.
[26] Q. G. Zhai, C. Z. Lu, S. M. Chen, X. J. Xu, W. B. Yang. A novel 3D hybrid architecture based on (H2O)6 encircling Cu4I4-O-Cu4I4 cluster and hexanuclear Cu6(datrz)6 ring. Inorg. Chem. Commun. 2006, 9, 819-822.
[27] R.-L. Sang, L. Xu. Supramolecular architectures built of biimidazole complexes and sulfate or nitrate anions. Polyhedron 2006, 25, 2167-2174.
[28] W. H. Zhu, Z. M. Wang, S. Gao. A 3D porous lanthanide-fumarate framework with water hexamer occupied cavities, exhibiting a reversible dehydration and rehydration procedure. Dalton Tran. 2006, 765-768.
[29] Y. C. Liao, Y. C. Jiang, S. L. Wang. Discrete Water Hexamers and Template-Assisted Molecular Recognition in an Elastic Zincophosphate Lattice. J. Am. Chem. Soc. 2005, 127, 12794-12795.
[30] A. Michaelides, S. Skoulika, E. G. Bakalbassis, J. Mrozinski. Cyclic Water Hexamers and Decamers in a Porous Lanthanide-Organic Framework: Correlation between Some Physical Properties and Crystal Structure. Cryst. Growth Des. 2003, 3, 487-492.
[31] J. N. Moorthy, R. Natarajan, P. Venugopalan. Characterization of a planar cyclic form of water hexamer in an organic supramolecular complex: an unusual self-assembly of bimesityl-3,3'-dicarboxylic acid. Angew. Chem., Int. Ed. 2002, 41, 3417-3420.
[33] S. K. Ghosh, P. K. Bharadwaj. Puckered-Boat Conformation Hexameric Water Clusters Stabilized in a 2D Metal-Organic Framework Structure Built from Cu(II) and 1,2,4,5-Benzenetetracarboxylic Acid. Inorg. Chem. 2004, 43, 5180-5182.
[34] B. H. Ye, B. B. Ding, Y. Q. Weng, X. M. Chen. Formation of One-Dimensional Metal-Water Chain Containing Cyclic Water Hexamers. Inorg. Chem. 2004, 43, 6866-6868.
[36] U. Mukhopadhyay, I. Bernal. Self-Assembled Hexameric Water Clusters Stabilized by a Cyano-Bridged Trimetallic Complex. Cryst. Growth Des.2005, 5, 1687-1689.
[37] R. J. Doedens,E. Yohannes, M. I. Khan. Novel water clusters in the crystalline state: structures of a symmetrical, cyclic hexamer and an ‘opened-cube’ octamer. Chem. Commun. 2002, 62-63.
[38] S. W. Benson, E. D. Siebert.A simple two-structure model for liquid water.J. Am. Chem. Soc. 1992, 114, 4269-4276.
[39]A. Hossein, G. Mohammad, M. Faranak, N. Bahar. A proton transfer compound of piperazine with pyridine-2,6-dicarboxylic acid and its palladium(II) and thallium(III) complexes - synthesis, characterization and crystal structure. Z. Anorg. Allg. Chem. 2006, 632, 2058-2064.
[40]F. N. Keutsch, J. D. Cruzan, R. J. Saykally. The Water Trimer. Chem. Rev. 2003, 103, 2533-2577.
[41] L. R. MacGillivray, J. L. Atwood. Molecular recognition of the cyclic water trimer in the solid state. J. Am. Chem. Soc. 1997, 119, 2592-2593.
[42] L.-N. Zhu, O.-Y. Yan, Z.-Q. Liu, D.-Z. Liao, Z.-H. Jiang, S.-P. Yan, P. Cheng. A novel mixed-ligand manganese(II) complex containing a V-shape water trimer: Synthesis, structure and magnetic properties of {[Mn(azpy)2(dca)(H2O)2](ClO4)(azpy)(H2O)2}n. Z. Anorg. Allg. Chem. 2005, 631, 1693-1697.
[43]C. H. Gorbitz. Cyclic water pentamers in L-leucyl-L-alanine tetrahydrate. Acta Cryst.C 1997, C53, 736-739.
[44]M. H. Mir, J. J. Vittal. Single-Crystal to Single-Crystal Transformation of Cyclic Water Heptamer to Another (H2O)7 Cluster Containing Cyclic Pentamer, Cryst. Growth Des. 2008, 8, 1478–1480.
[45] T. K. Prasad, M. V. Rajasekharan. A Novel Water Octamer in Ce(dipic)2(H2O)3·4H2O: Crystallographic, Thermal, and Theoretical Studies. Cryst. Growth Des. 2006, 6, 488-491.
[46] B. Q. Ma, H. L. Sun, S. Gao. Observation of an octameric water cluster containing a book-shaped hexamer in a 4f-3d complex. Chem. Commun. 2005, 2336-2338.
[47] S. K. Ghosh, P. K. Bharadwaj. Octameric Water Clusters of Staircase Structure Present in a Metal-Organic Framework Built from Helical Lanthanide Coordination Polymers. Eur. J. Inorg. Chem. 2005, 4886-4889.
[48] J. L. Atwood, L. J. Barbour, T. J. Ness, C. L. Raston, P. L. Raston. A Well-Resolved Ice-like (H2O)8 Cluster in an Organic Supramolecular Complex. J. Am. Chem. Soc. 2001, 123, 7192-7193.
[49] W. B. Blanton, S. W. Gordon-Wylie, G. R. Clark, K. D. Jordan, J. T. Wood, U. Geiser, T. J. Collins. Synthesis and Crystallographic Characterization of an Octameric Water Complex, (H2O)8. J. Am. Chem. Soc,1999, 121, 3551-3552.
[50]C. P. Pradeep, S. Supriya, P. S. Zacharias, S. K. Das. A tetra-nuclear copper(II) complex stabilizes an extended structure of a water nonamer: Synthesis and characterization of [Cu4(C54H46N4O14)(OH)2]·10H2O. Polyhedron 2006, 25, 3588-3592.
[51] S. K. Ghosh, P. K. Bharadwaj. Metal-Organic Framework H-Bonded Like a Polycatenane: Coexistence of Acyclic Water Trimer and Nonamer. Inorg. Chem. 2005, 44, 5553-5555.
[52]L. J. Barbour, G. W. Orr, J. L. Atwood. Anintermolecular (H2O)10 cluster in a solid-state supramolecular complex. Nature, 1998, 393, 671-673.
[53] L. J. Barbour, G. W. Orr, J. L. Atwood. Characterization of a well resolved supramolecular ice-like (H2O)10 cluster in the solid state. Chem. Commun. 2000, 859-860.
[54] Y. Li, L. Jiang, X.-L. Feng, T.-B. Lu. Formation of a Decameric Water Ring in a Cryptand-Phthalic Acid Supramolecular Adduct. Cryst. Growth Des. 2006, 6, 1074-1077.
[55] R. D. Bergougnant, A. Y. Robin, K. M. Fromm. “Hooked-on” Calix[8]arenes: A (H2O)10 Cluster with an Unprecedented Structure. Cryst. Growth Des.2005, 5, 1691-1694.
[56] M. Yoshizawa, T. Kusukawa, M. Kawano, T. Ohhara, I. Tanaka, K. Kurihara, N. Niimura, M. Fujita. Endohedral Clusterization of Ten Water Molecules into a “Molecular Ice” within the Hydrophobic Pocket of a Self-Assembled Cage. J. Am. Chem. Soc. 2005, 127, 2798-2799.
[57] P. S. Lakshminarayanan, E. Suresh, P. Ghosh.A Hybrid Water-Chloride Structure with Discrete Undecameric Water Moieties Self-Assembled in a Heptaprotonated Octaamino Cryptand.Angew. Chem. Int. Ed. 2006, 45, 3807-3811.
[58] S. K. Ghosh, P. K. Bharadwaj. A dodecameric water cluster built around a cyclic quasiplanar hexameric core in an organic supramolecular complex of a cryptand. Angew Chem., Int. Ed. 2004, 43, 3577-3580.
[59] S. Neogi, G. Savitha, P. K. Bharadwaj. Structure of Discrete (H2O)12 Clusters Present in the Cavity of Polymeric Interlinked Metallocycles of Nd(III) or Gd(III) and a Podand Ligand. Inorg. Chem.2004, 43, 3771-3773.
[60] M. S. Deshpande, A. S. Kumbhar, V. G. Puranik, K. Selvaraj. Supramolecular Self-Assembled Ruthenium-Polypyridyl Framework Encapsulating Discrete Water Cluster. Cryst. Growth Des. 2006, 6, 743-748.
[61] S. K. Ghosh, J. Ribas, M. S. E. Fallah, P. K. Bharadwaj. Mn(II) Staircase Structures Stitched by Water Clusters to a 3D Metal-Organic Open Framework: X-ray Structural and Magnetic Studies. Inorg. Chem. 2005, 44, 3856-3862.
[62] S. K. Ghosh, P. K. Bharadwaj. Structure of a Discrete Hexadecameric Water Cluster in a Metal-Organic Framework Structure. Inorg. Chem. 2004, 43, 6887-6889.
[63] L. Beitone, C. Huguenard, A. Gansmüller, M. Henry, F. Taulelle, T. Loiseau, G. Férey. Order-Disorder in the Super-Sodalite Zn3Al6(PO4)12, 4tren, 17H2O (MIL-74): A Combined XRD-NMR Assessment.J. Am. Chem. Soc.2003, 125, 9102-9110.
[64] C. H. Li, K. L. Huang, J. M. Dou, Y. N. Chi, Y. Q. Xu, L. Shen, D. Q. Wang, C. W. Hu. An Interesting Six-Connected 3D Nanowater Framework Constructed from Turbine-Type (H2O)18 Clusters Based on a Mn(III) Complex. Cryst. Growth Des. 2008, 8, 3141-3143.
[65] Y. N. Chi, K. L. Huang, S. W. Zhang, F. Y. Cui, Y. Q. Xu, C. W. Hu. Self-Assembly of a CsCl-like 3D Supramolecular Network from [Zn6(HL)6(H2L)6] Metallamacrocycles and (H2O)20 Clusters (H2L = 4-(2-Pyridyl)-6-(4-pyridyl)-2-aminopyrimidine). Cryst. Growth Des. 2007, 7, 2449–2453.
[66] M. L. Wei, C. He, W. J. Hua, C. Y. Duan, S. H. Li, Q. J. Meng, A Large Protonated Water Cluster H(H2O)27 in a 3D Metal-Organic Framework. J. Am. Chem. Soc. 2006, 128, 13318-13319.
[67] G. Sainz, C. J. Carrell, M. V. Ponamerev, G. M. Soriano, W. A. Cramer, J. L. Smith. Interruption of the Internal Water Chain of Cytochrome f Impairs Photosynthetic Function. Biochemistry. 2000, 39, 9164-9173.
[68] E. Tajkhorshid, P. Nollert, M. O. Jensen, L. J. W. Miercke, J. O’Connell, R. M. Stroud, K. Schulten. Control of the selectivity of the aquaporin water channel family by global orientational tuning. Science 2002, 296, 525-530.
[69] L. E. Cheruzel, M. S. Pometun, M. R. Cecil, M. S. Mashuta, R. J. Wittebort, R. M. Buchanan. Structures and Solid-State Dynamics of One-Dimensional Water Chains Stabilized by Imidazole Channels. Angew Chem., Int. Ed. 2003, 42, 5452-5455.
[70] X. L. Zhang, B. H. Ye, X. M. Chen. Infinite Water Chains Trapped in an Organic Framework Constructed from Melamine with 1,5-Naphthalenedisulfonic Acid via Hydrogen Bonds. Cryst. Growth Des. 2005, 5, 1609-1616.
[71] Z. H. Zhou, J. M. Yang, H. L. Wan. Diamine Substitution Reactions of Tetrahydrate Succinato Nickel, Cobalt, and Zinc Coordination Polymers. Cryst. Growth Des. 2005, 5, 1825-1830.
[72] D. L. Reger, R. F. Semeniuc, C. Pettinari, F. Luna-Giles, M. D. Smith. Structural Impact of Infinite Water Chains on the Self-Assembly of an Inorganic-Metal-Organic Architecture. Cryst. Growth Des. 2006, 6, 1068-1070.
[73] H.-J. Kim, H. J. Jo, J. Kim, S. Y. Kim, D. Kim, K. Kim. Supramolecular self-assembly of tin(IV) porphyrin channels stabilizing single-file chains of water molecules. CrystEngComm. 2005, 7, 417-420.
[74] B. Sreenivasulu, J. J. Vittal. Helix inside a Helix: Encapsulation of Hydrogen-Bonded Water Molecules in a Staircase Coordination Polymer. Angew. Chem., Int. Ed.2004, 43, 5769-5772.
[75] A. Mukherjee, M. K. Saha, M. Nethaji, A. R. Chakravarty. Helical supramolecular host with aquapores anchoring alternate molecules of helical water chains. Chem. Commun. 2004, 716-717.
[76] B. K. Saha, A. Nangia.Helical water chains in aquapores of organic hexahost: remarkable halogen-substitution effect on the handedness of water helix. Chem. Commun. 2005, 3024-3026.
[77] X. L. Zhang, X. M. Chen. Supramolecular Architectures and Helical Water Chains in Cocrystals of Melamine and Aromatic Carboxylic Acids. Cryst. Growth Des. 2005, 5, 617-622.
[78] B. Y. Lou, F. L. Jiang, D. Q. Yuan, B. L. Wu, M. C. Hong. Synthesis and Characterization of a 3D H-Bonded Supramolecular Complex with Chiral Channels Encapsulating 1D Left-Handed Helical Water Chains. Eur. J. Inorg. Chem. 2005, 3214-3216.
[79] B. H. Ye, A. P. Sun, T. F. Wu, Y. Q. Weng, X. M. Chen. Tapes of Cyclic Water Tetramers in the Double-Helical Complex [Cd2(bpa)2Cl4]·6H2O. Eur. J. Inorg. Chem. 2005, 1230-1234.
[80] J. M. Zheng, S. R. Batten, M. Du. A Unique Cyanide-Bridged Three-Dimensional (3-D) Copper(II)-Copper(I) Mixed-Valence Polymer Containing 1-D Water Tapes with Cyclic Pentamer Units. Inorg. Chem. 2005, 44, 3371-3373.
[81]R. Luna-García, B. M. Damián-Murillo, V. Barba, H. Höpfl, H. I. Beltrán, L. S. Zamudio-Rivera.Structural relationship between a host included chain of spirocyclic water hexamers and bulk water-the role of water clusters in self assembly and crystallization processes. Chem. Commun.2005, 5527-5529.
[82] B. Q. Ma, H. L. Sun, S. Gao. Vertex-Sharing Water Tape Consisting of Cyclic Hexamers. Eur. J. Inorg. Chem. 2005, 3902-3906.
[83] B. K. Saha, A. Nangia. First example of an ice-like water hexamer boat tape structure in a supramolecular organic host. Chem. Commun.2006, 1825-1827.
[84] X. M. Zhang, R. Q. Fang, H. S. Wu. Extended Water Tapes of Cyclic Hexamers Encapsulated in the Channels of a Metal Phosphonocarboxylate Network. Cryst. Growth Des. 2005, 5, 1335-1337.
[85] R. Custelcean, C. Afloroaei, M. Vlassa, M. Polverejan. Formation of Extended Tapes of Cyclic Water Hexamers in an Organic Molecular Crystal Host. Angew. Chem., Int. Ed. 2000, 39, 3094-3096.
[86] A.Wakahara, T. Ishida. Novel 1-D water nanowires in crystal of an organic host.Chem. Lett.2004, 33, 354-355.
[86]J. Lu, J.-H. Yu, X.-Y. Chen, P. Cheng, X. Zhang, J.-Q. Xu. Novel Self-Assembled Chain of Water Molecules in a Metal-Organic Framework Structure of Co(II) with Tartrate Acid. Inorg. Chem. 2005, 44 (17), 5978-5980.
[87] M. Tadokoro, S. Fukui, T. Kitajima, Y. Nagao, S. Ishimaru, H. Kitagawa, K. Isobe, K. Nakasuji. Structures and phase transition of multi-layered water nanotube confined to nanochannels. Chem. Commun.2006, 1274-1276.
[88] S. Q. Zang, Y. Su, C. Y. Duan, Y. Z. Li, H. Z. Zhu, Q. J. Meng. Coexistence of chiral hydrophilic and achiral hydrophobic channels in one multi-helical-array metal-organic framework incorporating helical water cluster chains. Chem. Commun.2006, 4997-4999.
[89] D. L. Reger, R. F. Semeniuc, C. Pettinari, F. Luna-Giles, M. D. Smith. Structural Impact of Infinite Water Chains on the Self-Assembly of an Inorganic-Metal-Organic Architecture. Cryst. Growth Des. 2006, 6, 1068-1070.
[90] N. H. Hu, Z. G. Li, J. W. Xu, H. Q. Jia, J. J. Niu. Self-Assembly of a Water Chain with Tetrameric and Decameric Clusters in the Channel of a Mixed-Valence CuCu Complex. Cryst. Growth Des. 2007, 7, 15-17.
[91]S. Neogi, P. K. Bharadwaj. An Infinite Water Chain Passes through an Array of Zn(II) Metallocycles Built with a Podand Bearing Terminal Carboxylates. Inorg. Chem. 2005, 44, 816-818.
[92] F. Li, T. H. Li, D. Q. Yuan, J. Lv, R. Cao. Characterization of a novel water tape containing (H2O)18 clusters. Inorg. Chem. Commun. 2006, 9, 691-694.
[93] S. R. Choudhury, A. D. Jana, E. Colacio, H. M. Lee, G. Mostafa, S. Mukhopadhyay. Crowned tetrameric spirocyclic water chain: an unusual building block of a supramolecular metal-organic host. Cryst. Growth Des.2007, 7, 212-214.
[94]K. M. Park, R. Kuroda, T. Iwamoto. A two-dimensional ice with the topography of edge-sharing hexagons intercalated between CdNi(CN)4 layers. Angew. Chem., Int. Ed. 1993, 32, 884-886.
[95] B. Q. Ma, H. L. Sun, S. Gao. Formation of Two-Dimensional Supramolecular Icelike Layer Containing (H2O)12 Rings.Angew. Chem. Int. Ed. 2004, 43, 1374-1376.
[96] P. Rodríguez-Cuamatzi, G. Vargas-Díaz, H. Höpfl.Modification of 2D Water That Contains Hexameric Units in Chair and Boat Conformations-A Contribution to the Structural Elucidation of Bulk Water. Angew. Chem. Int. Ed. 2004, 43, 3041-3044.
[97] X. J. Luan, Y. C. Chu, Y. Y. Wang, D. S. Li, P. Liu, Q. Z. Shi. Formation of Two-Dimensional Supramolecular Water Layer Containing (H2O)18 Morphology via Dianion Templating. Cryst. Growth Des. 2006, 6, 812-814.
[98]R. Carballo, B. Covelo, N. Fernández-Hermida, E. García-Martínez, A. B. Lago, M. Vázquez, E. M. Vázquez-López.Supramolecular Aggregation of Hexameric Water Clusters into a 2D Water Polymer Containing (H2O)18 Holes.Cryst. Growth Des. 2006, 6, 629-631.
[99]N. S. Oxtoby, A. J. Blake, N. R. Champness, C. Wilson.Water Superstructures within Organic Arrays, Hydrogen-Bonded Water Sheets, Chains and Clusters. Chem. Eur. J. 2005, 11, 4643-4654.
[100] J. P. Zhang, Y. Y. Lin, X. C. Huang, X. M. Chen. Well-Resolved, New Water Morphologies Obtained by Modification of the Hydrophilic/Hydrophobic Character and Shapes of the Supporting Layers. Inorg. Chem. 2005, 44, 3146-3150.
[101]Z. F. Fei, T. J. Geldbach, D. B. Zhao, R. Scopelliti, P. J. Dyson. A Nearly Planar Water Sheet Sandwiched between Strontium-Imidazolium Carboxylate Coordination Polymers. Inorg. Chem.2005, 44, 5200-5202.
[102]C. Janiak, T. G. Scharmann.Two-Dimensional Water and Ice Layers: Neutron Diffraction Studies at 278, 263, and 20 K. J. Am. Chem. Soc. 2002, 124, 14010-14011.
[103] P. S. Lakshminarayanan, E. Suresh, P. Ghosh. Formation of an Infinite 2D-Layered Water of (H2O)45 Cluster in a Cryptand-Water Supramolecular Complex: A Template Effect.J. Am. Chem. Soc,2005, 127, 13132 -13133.
[104] R. Carballo, B. Covelo, C. Lodeiro, E. M. Vázquez-López. One-dimensional fluorescent stacking structure based on zinc mixed-complex salt encapsulated within an ‘ice-like’ three-dimensional hydrogen-bonded water network. CrystEngComm. 2005, 7, 294-296.
[105] M. Mascal, L. Infantes, J. Chisholm. Water Oligomers in Crystal Hydrates-What's News and What Isn't? Angew. Chem. Int. Ed.2006, 45, 32-36.
[106] Y. T. Wang, G. M. Tang, Z. M. Liu, X. H. Yi. Can One-Dimensional Water Be Controlled by Transformation of Substitution Groups Based on Organic Hosts? Cryst. Growth Des. 2007, 7, 2272–2275.
[107]L. Cheng, J. B. Lin, J. Z. Gong, A. P. Sun, B. H. Ye, X. M. Chen. Encapsulation of Water Cluster, meso-Helical Chain and Tapes in Metal-Organic Frameworks Based on Double-Stranded Cd(II) Helicates and Carboxylates. Cryst. Growth Des. 2006, 6(12), 2739-2746.
[108] S.-p. Chen, M. Li, Y. Xiao, Y.-x. Yuan, L.-l. Pan, L.-j. Yuan, Hydrogen-bonded assembly of [Ni(Im)6] ion and phosphorus anions: Different sandwiched-type/tessellate-type supramolecular architectures and 1D water chains, CrystEngComm 2008, 10, 1227–1236.
[109] S.-p. Chen, G.-x. Huang, M. Li, L.-l. Pan, Y.-x. Yuan, L.-j. Yuan, New in Situ Condensation Reaction of Amino Dphosphonic Acids: A Series of Bicyclic Phosphonate Derivatives and Three Novel Water Clusters, Cryst. Growth Des.2008, 8, 2824–2833
[110]S.-p. Chen, Y.-x. Yuan, L.-l. Pan, L.-j. Yuan, Vertical Interpenetra -tion of 1D Water Column and 1D Coordination Polymer Chain,J. Inorg. Organomet. Polym. 2008, 18, 384–390.